Pediatric Multimodal Neuroimaging
Neuroimaging techniques are an important tool to identify altered structures or functions in the brain and to infer pathological processes from them. In multimodal neuroimaging, data from different neuroimaging techniques are combined to obtain more extensive and detailed information.
Research Focus
In vivo CSF Flow Dynamics
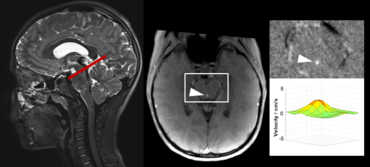
Recent studies of cerebrospinal fluid (CSF) circulation, pathways and dynamics have rigorously challenged century-old classical views.
Advanced, ultra-fast techniques like real-time phase-contrast (RT-PC) flow MRI have facilitated new insights into CSF flow, its physiology and driving forces. Advantage of the RT MRI method is its independence of any physiological gating and enables CSF flow measurements without e.g. ECG trigger. Our studies of CSF dynamics identified forced inspiration as the dominant driver that prompts an upward surge of CSF from the lumbar region into the cranial vault and towards the brain ventricular spaces.
However, we still know little about how disturbances of CSF circulation lead to diseases like hydrocephalus and syringomyelia in particular in children. Hence, studies of regulatory forces of CSF dynamics are of imminent clinical importance to better understand the disease processes and, more importantly, to derive the most specific therapeutic strategies. Our working group applies RT flow MRI to investigate the influence of respiration/cardiac pulse on interplay between CSF and venous fluids to establish age dependent control values and as a second step in patients with disturbed CSF circulation.
Coupling mechanisms of CSF and venous flow dynamics
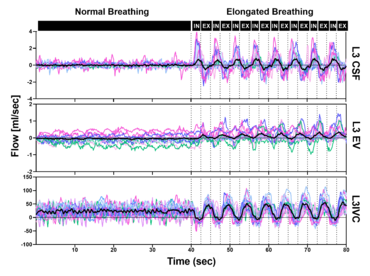
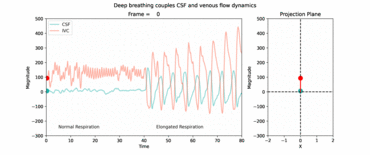
RT flow MRI revealed forced inspiration as the dominant driver that prompts an upward surge of CSF. Concomitantly, venous drainage out of the head increases governed by the inspiratory lowering of the intrathoracic pressures. These latest insights point to an equilibrium between CSF and venous systems and its essential role for the regulation of intracranial volume and pressure. Our studies showed that deep respiration represents one coupling mechanism of interdependent venous and brain fluid flow. The clinical impact is focus of our research, as increasing numbers of clinical observations imply an important pathophysiological role. For example, compromised venous flow is considered one major cause of idiopathic intracranial hypertension.
Knowledge about the regulatory forces and interdependency of CSF and blood flow dynamics is of great importance to elucidate disease processes of pediatric hydrocephalus and disturbances of CSF circulation. Furthermore, our working is exploring how the neurofluid systems (arterial flow, venous flow and CSF flow) are interconnected to each other.
Click here or figure 2 for animation.
Advanced multimodal quantitative MR Imaging in White Matter Disorders
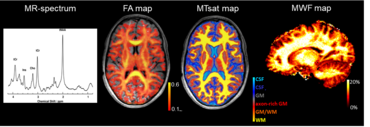
Our ongoing work focuses on application of advanced quantitative MRI techniques for myelin imaging in a wide range of neurological diseases in pediatric patients. More specifically, we aim to develop reliable imaging markers for monitoring neurodegeneration and repair mechanisms in childhood white matter disorders. The comprehensive age-dependent multiparametric MRI protocol comprises e.g. MR-spectroscopy, DTI and novel analysis methods such as neurite orientation dispersion and density imaging (NODDI). The parameters obtained by these methods are sensitive to microstructural alterations of the different brain tissue structures such as axons and myelin sheath. Extending the protocol further for myelin-sensitive MRI we employ magnetization transfer imaging (MTI) as well as the innovative multicomponent myelin water imaging (MWI) (mcDESPOT) which up to today possesses the highest specificity for myelin. Furthermore, our group explores the potential of myelin-sensitive quantitative MRI parameter as biomarkers to monitor the effects of myelin modulating treatments. The combination of MRI techniques may allow to investigate individual myelination processes and thus provide a basis for therapeutic decisions and evaluation of therapy success.
Brain networks in pediatric multiple sclerosis studied by multimodal MRI
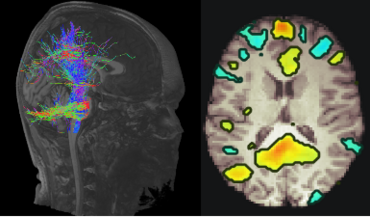
In current clinical practice, MR Imaging is used to evaluate macroscopic structural changes for i) diagnosis of neuroinflammatory and neurometabolic diseases in the pediatric patients such as Multiple Sclerosis (MS) and X-linked adrenoleukodystrophy and ii) assessing disease progression. However, advanced quantitative MRI techniques visualize microstructural changes in brain tissue and facilitate the identification of functional and structural brain networks. Up to this day, these methods are not commonly applied in clinical settings. Neuronal networks form the basis for brain function. They undergo maturation processes during childhood and adolescence and show pathological changes in neurodegenerative diseases. Pediatric patients with MS suffer from inflammatory demyelinating attacks during the initial stages of the disease and later in adulthood they experience progressive neurodegeneration. However, recent studies suggest, that neurodegeneration already begins in adolescence. The effects of neurodegenerative diseases on function and maturation of brain networks in pediatric patients are vastly unknown. Our goal is to investigate large scale brain networks of pediatric patients with MS using MR Imaging in a longitudinal study design. Aim is to characterize the interaction between physiological maturation processes and neurodegenerative processes at the network level.
Publications
Dreha-Kulaczewski, S. et al. Inspiration is the major regulator of human CSF flow. J Neurosci 35, 2485-91 (2015).
Dreha-Kulaczewski S, Joseph AA, Merboldt K-D, Ludwig H-C, Gärtner J, Frahm J. Identification of the Upward Movement of Human CSF In Vivo and its Relation to the Brain Venous System. J Neurosci Off J Soc Neurosci. United States; 2017;37:2395–402.
Bock, H.C., Dreha-Kulaczewski, S.F., Alaid, A., Gartner, J. & Ludwig, H.C. Upward movement of cerebrospinal fluid in obstructive hydrocephalus-revision of an old concept. Childs Nerv Syst 35, 833-841 (2019).
Kollmeier JM, Gürbüz-Reiss L, Sahoo P, Badura S, Ellebracht B, Keck M, et al. Deep breathing couples CSF and venous flow dynamics. Sci Rep. England; 2022;12:2568.
Dreha-Kulaczewski SF, Brockmann K, Henneke M, Dechent P, Wilken B, Gärtner J, Helms G. Assessment of myelination in hypomyelinating disorders by quantitative MRI. J Magn Reson Imaging 2012, 36:1329-1338.
Cooperations
Prof. Jens Frahm, Biomedical NMR, Max Planck Institute for Multidisciplinary Sciences, Göttingen
PD Dr. Christoph Bock, Prof. Frederike Knerlich-Lukoschus, Pediatric Neurosurgery, University Medicine Göttingen
PD Dr. P. Dechent, Dr. Carsten Schmidt-Samoa MR Research in Neuroscience, Göttingen
Prof. Dr. H. Riedel, Institut für Diagnostische und Interventionelle Neuroradiologie, University Medicine Göttingen
PD Dr. Hagen Kitzler, Caroline Köhler, Paul Kuntke, Research Group White Matter Imaging, Institut für Diagnostische und Interventionelle Neuroradiologie, Universitätsklinikum Carl Gustav Carus, Technische Universität Dresden
Sean Deoni, PhD, School of Engineering Brown University, Providence, RI, USA
Gunther Helms, PhD, Lund University, Sweden
Prof. Uwe Klose, Abteilung für Diagnostische und Interventionelle Neuroradiologie, Universitätsklinik Tübingen
Funding
Deutsche Forschungsgemeinschaft TRR274
Mrs. L. Grun funds
ELA Deutschland
Principal Investigators
Priv.-Doz. Dr. med. Steffi Dreha-Kulaczewski

Steffi Dreha-Kulaczewski started to work in MR-Research in the Departments of Radiology and Neuroradiology at UPENN and CHOP, Philadelphia, PA, USA. She completed her doctoral and PhD thesis in medicine with Prof. Gärtner, University Medicine Goettingen, applying multimodal advanced MR Imaging to various childhood white matter disorders. The comprehensive quantitative MRI study protocol enables assessments of de- and remyelinating processes as well as axonal involvement. Goals are the development of reliable imaging markers for monitoring myelin modulating therapies e.g. in cerebral folate deficiency.
In collaboration with Prof. Jens Frahm from the MPI in Goettingen, she studies CSF dynamics using real-time phase-contrast MRI in healthy conditions and normal development. Steffi investigates coupling mechanisms of CSF and cerebral venous flow as aspects of the novel neurofluids concepts. Aims are to better understand underlying pathophysiologic processes of childhood hydrocephalus and facilitate more specific therapies.
Contact: s.dreha(at)med.uni-goettingen.de
Dr. rer. nat. Prativa Sahoo

Dr. Sahoo has completed her PhD in the department of Mathematica and statistics at Indian institute of technology Kanpur, India under supervision of Prof. RKS Rathore. Her doctoral thesis was focused on pharmacokinetic modeling for glioma patients using dynamic contrast enhanced (DCE) MRI. She extended her knowledge in the field of quantitative MRI developing predictive model for response to treatment, implementing post-processing tools, optimizing imaging protocol at Philips Healthcare, India, and Beckman Research Institute, City of Hope, CA, USA. She is a research scientist at Universitätsmedizin Göttingen in the department of Pediatrics and Adolescent Medicine. Here she is involved in various projects such as finding imaging biomarkers for early detection of Leukodystrophy (LD), exploring mechanism of CSF circulation and its relation to respiration.
Contact: psahoo(at)gwdg.de
Research Interest:
Quantitative MRI techniques: 4D flow real-time phase contrast MRI, Dynamic Contrast enhanced MRI (DCE), diffusion tensor Imaging (DTI)
Data driven Mathematical and computational model: predictive model for cancer growth and response to treatment, pharmacokinetic modeling, fluid dynamics.
Dr. med. Simon Badura

Simon Badura obtained his PhD in medicine in the lab of Prof. Staiger at the institute of neuroanatomy, Göttingen. During his doctoral work he was examining the short-term plasticity of cortical interneurons with whole-cell patch-clamp recordings. After the PhD he started with in vivo measurements of CSF and venous flow using real-time phase-contrast MRI. Since 2022 he participated at a clinical scientist program by the Eva Luise und Horst Köhler Stiftung für seltene Erkrankungen. His aims are examining ATP1A3 mutations with bioengineered neuronal organoids and induced human stem cell cultures.
Contact: simon.badura(at)med.uni-goettingen.de
Dr. med. Kolja Meier

Kolja Meier analyzed network activity in the mouse hippocampus during his PhD in medicine at the Center for Molecular Neurobiology in Hamburg in the research group Experimental Neuropediatrics of Prof. Dirk Isbrandt. After his PhD he began his residency in pediatrics in Göttingen. As early clinician scientist in the TRR274 he develops postprocessing pipelines for multimodal MRI data of patients with neuroinflammatory and neurometabolic diseases, such as pediatric MS and X-linked adrenoleukodystrophy. Furthermore, he is further engaged in the analysis of trio-exome data to solve cases of patients with unclear neurometabolic diseases.
Contact: kolja.meier(at)med.uni-goettingen.de
PhD/MD students
Nora Wenkel
Mathilda Maria Keck
Irini Gkalimani
Ben Ellebracht
Lukas Gürbüz-Reiß